Introduction: Patients with standard risk (SR) multiple myeloma (MM), as defined by the absence of high risk cytogenetic abnormalities including immunoglobulin heavy chain (IGH) gene translocations such as t(4;14), t(14;16), t(14;20), deletion of (17p), or gain/amplification(1q), have favorable prognosis and respond well to standard of care induction with plasma-cell directed therapy followed by AHCT consolida-tion and lenalidomide (LEN)-based maintenance. Despite this, it has been noted that a significant proportion of SRMM patients may suffer from early relapse after AHCT, and could potentially benefit form mitigation strategies. The aims of this study were to assess the impact of prior lines of therapy on post-AHCT outcomes in SR MM patients, and then evaluate the role of an augmented LEN-based maintenance regimen in those who did not achieve a complete response (CR) post transplant.
Methods: All MM patients who underwent AHCT at our institutions between January 2011 and January 2021 were retrospectively reviewed. Electronic medical charts were searched to collect data on baseline demographic characteristics, disease status, fluorescent in situ hybridization (FISH) cytogenetic studies, blood and bone marrow studies, number of lines of treatments pre-AHCT, as well as maintenance therapy post-transplant. Responses were according to the International Myeloma Working Group (IMWG) criteria. Kaplan Meier method was used for event free survival (EFS) assessment.
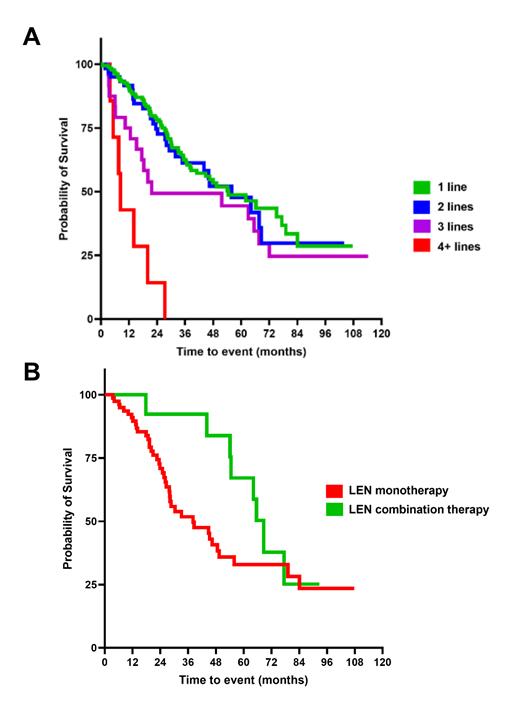
Results: A total of 293 SR MM patients were included in this analysis, of whom 188, 63, 32 and 10 received 1, 2, 3, and ≥4 lines of therapy pre-AHCT. Despite having comparable CR rates pre-AHCT (28% vs 19% vs 22% vs 10%, respectively; p=0.3), patients with ≥4 prior lines of therapy experienced significantly worse outcomes as determined by shorter mediant EFS of 8.4 months vs 54.5, 55.9 and 21.7 months (P<0.0001) for the 1, 2, and 3 pre-AHCT lines of therapy groups, respectively ( Figure 1A). We then focused on the subgroup of SR MM patients (n=100) who did not achieve a hematologic CR at day-100 post-AHCT. Among them 87 were placed on LEN-monotherapy, and 13 on LEN-based combination maintenance therapy post-AHCT. Of note, none was LEN-refractory. Both the LEN-monotherapy and combination groups had comparable baseline patients and disease characteristics including age (60.5 vs 56 years, p=0.07), time from diagnosis to transplant (≥12 months in 24% vs 30.8%, p=0.7), hematopoietic cell transplantation-specific comorbidity index (HCT-CI) score ( ≥3 in 44% vs 30.8%, p=0.5), male representation (59% vs 69%, p=0.6), white ethnicity (82 vs 77%, p=0.7), international scoring system stages (p=0.9), pre-AHCT number of lines of therapy (p=0.8), and pre-AHCT bone marrow plasma cell percentage (≥10% in 9.5% vs 7.7%, p=0.9), respectively. Kaplan-Meier survival analysis showed a significantly shorter median estimated EFS for the LEN monotherapy group at 38.1 months vs 68.6 months (p=0.03) for the LEN combination maintenance group ( Figure 1B).
Conclusion: SR MM constitute heterogenous group of patients with variable outcomes post-AHCT. Those with heavily pretreated disease, that have received four or more lines or therapy, tend to have worse outcomes post transplant, therefore, should be considered for alternative approaches such as chimeric antigen receptor T cell therapies or bispecific agents. For patients who undergo AHCT but do not achieve a CR, LEN-combination maintenance should be strongly considered especially in the setting of relapsed-refractory disease.
Disclosures
Sauter:Kite/a Gilead Company, Celgene/BMS, Gamida Cell, Karyopharm Therapeutics, Ono Pharmaceuticals, MorphoSys, CSL Behring, Syncopation Life Sciences, CRISPR Therapeutics and GSK.: Consultancy; Juno Therapeutics, Celgene/BMS, Bristol-Myers Squibb, Precision Biosciences, Actinium Pharmaceuticals, Sanofi-Genzyme and NKARTA.: Research Funding. Khouri:Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events; GPCR Therapeutics: Other: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events. Valent:Alexion, AstraZeneca Rare Disease: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal